CHEMISTRY & PHYSICAL SCIENCES
DEPARTMENT MENU
Welcome to Nicholls Chemistry & Physical Sciences
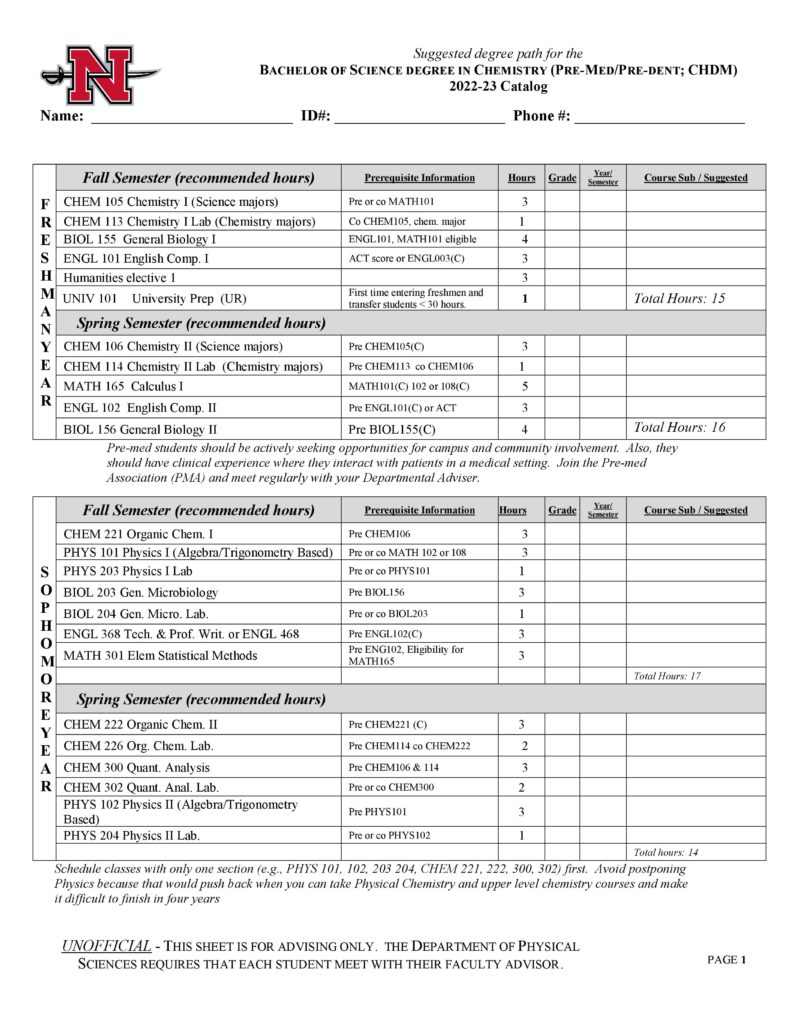
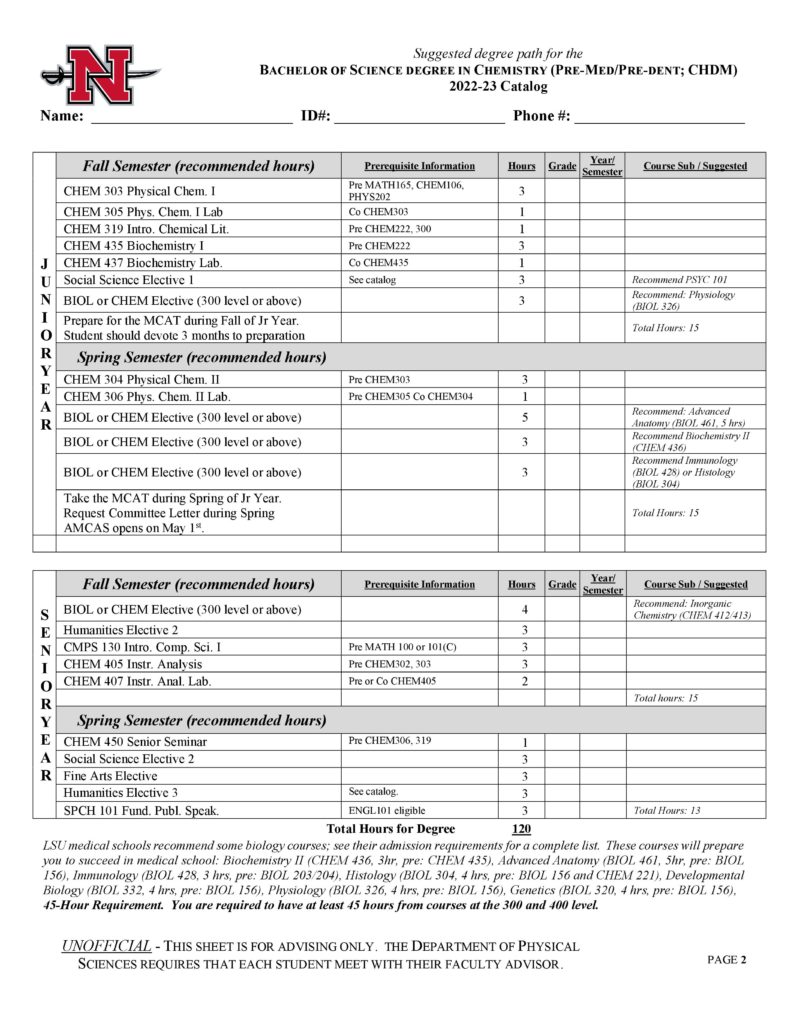
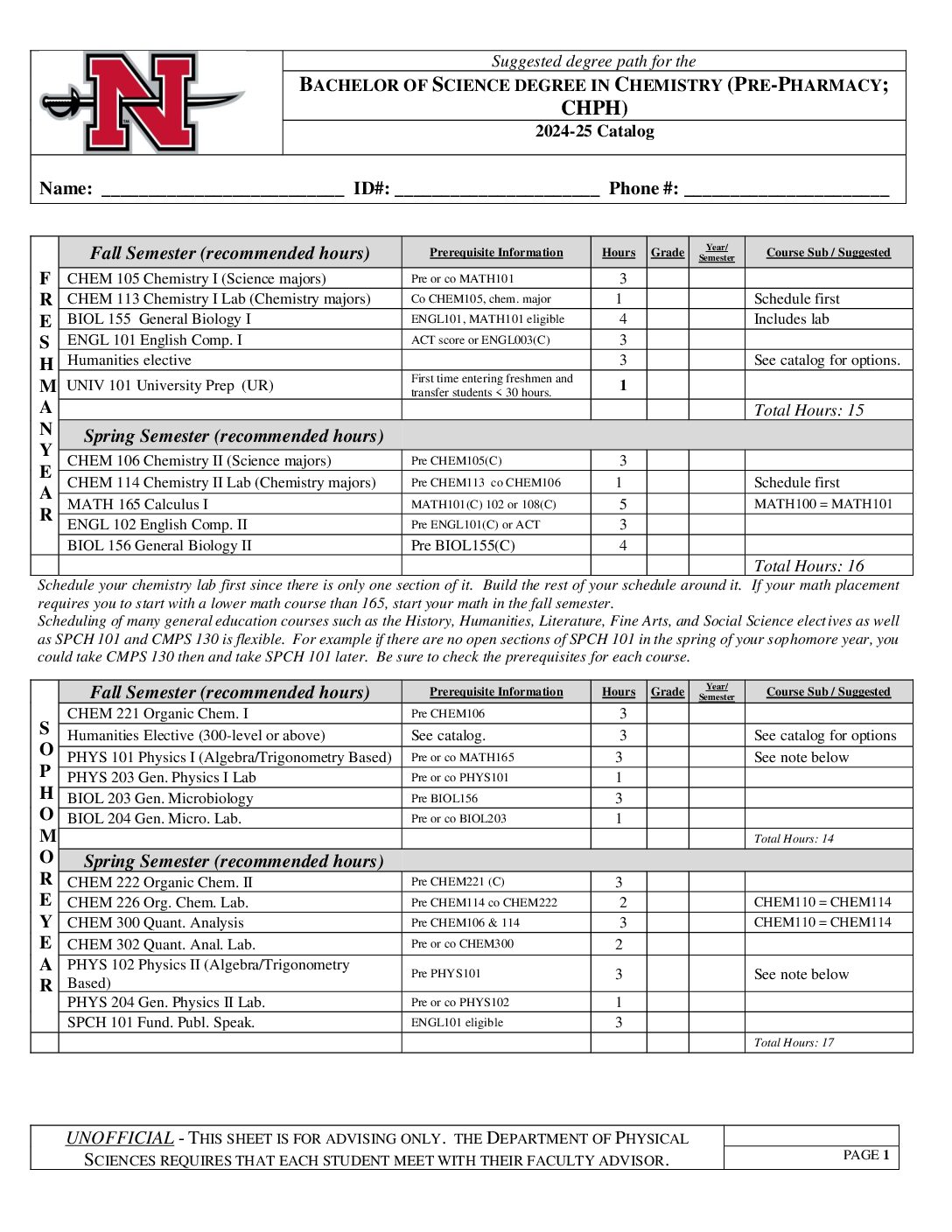
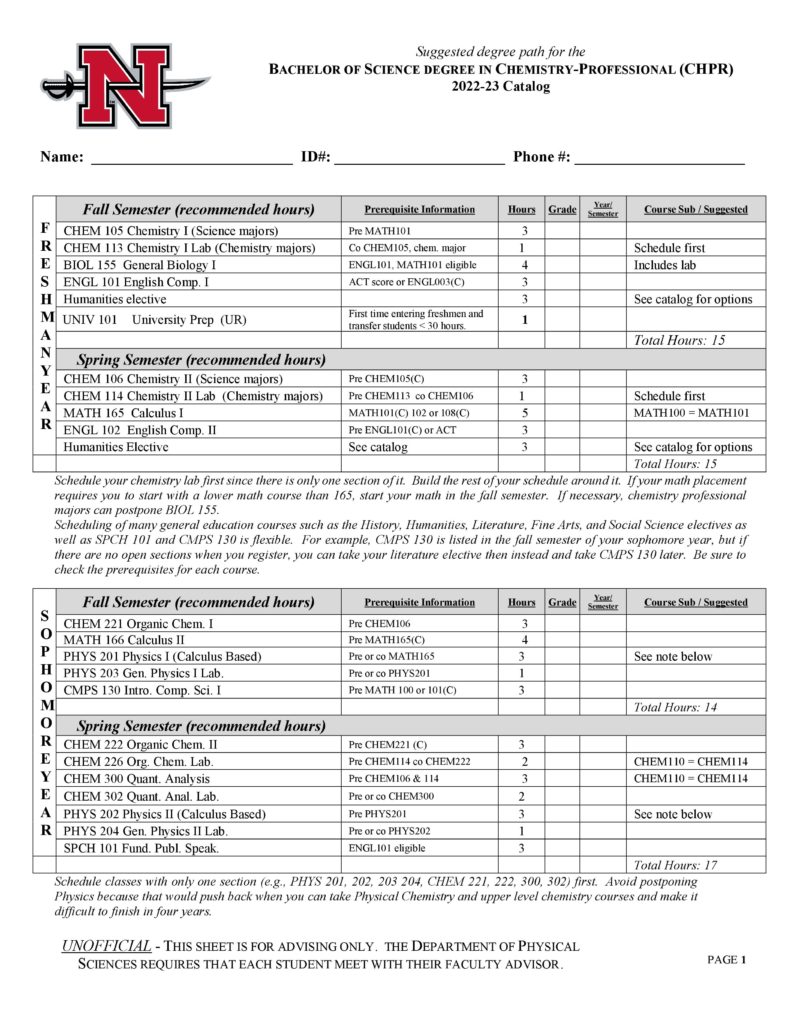
With concentrations available in professional chemistry, pre-med and pre-dental, pre-pharmacy, biochemistry and chemistry education, Nicholls State University is an ideal place to learn chemistry with its outstanding faculty and modern facilities. Undergraduate students participate in research with faculty, co-author papers, and present research at conferences. The program prepares students to work as chemists, doctors, dentists, teachers, pharmacists, and biochemists. Nicholls chemistry graduates are regularly accepted to graduate school and are highly sought after on the job market.
ALUMNI SPOTLIGHT

Thanks to Nicholls Chemistry, I was able to spend the summer in Russia studying microorganisms in permafrost, as part of a competitive undergraduate research program. It has given me experience and made me see what direction I want to go in after undergrad.
Alexandra Aucoin
ACCREDITATION

Nicholls chemistry is accredited by the American Chemical Society, which opens the door for graduate schools and careers in industry and teaching.
Preferred Admission
ACADEMICS


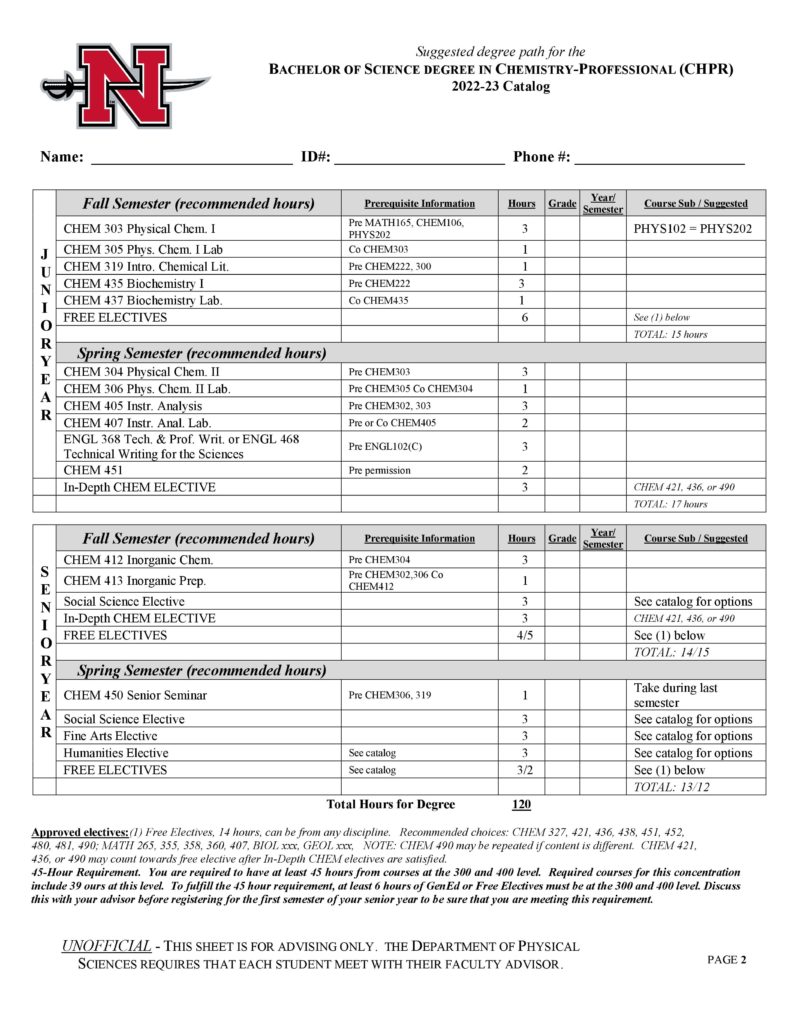
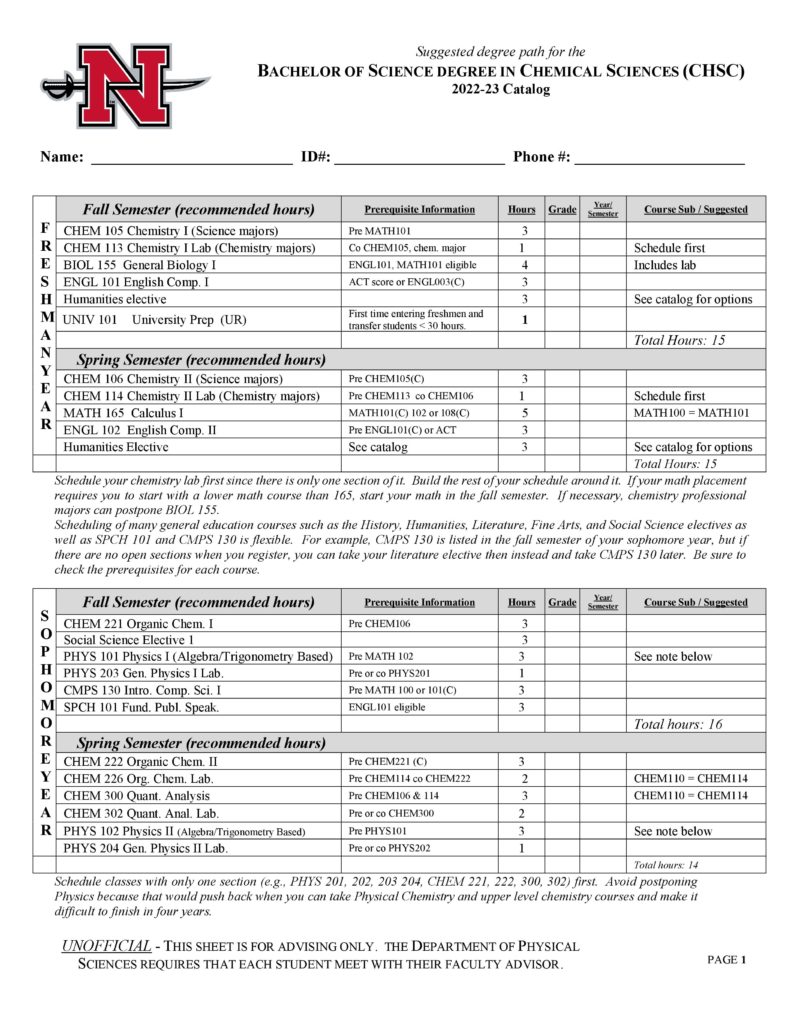
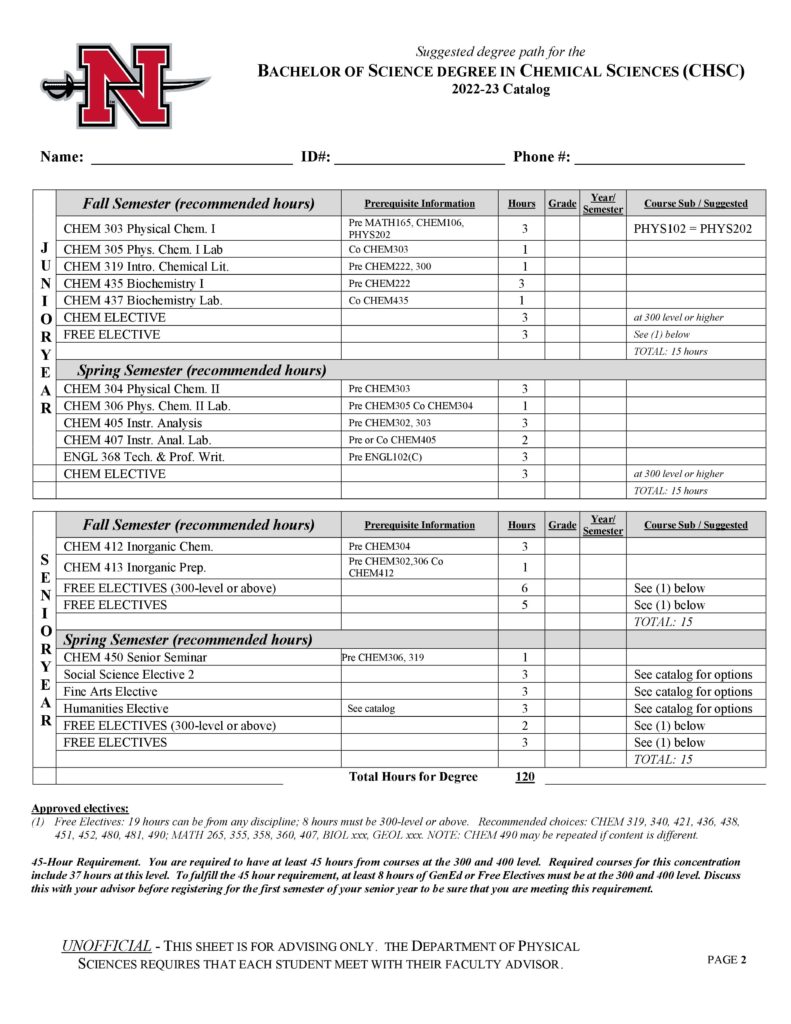
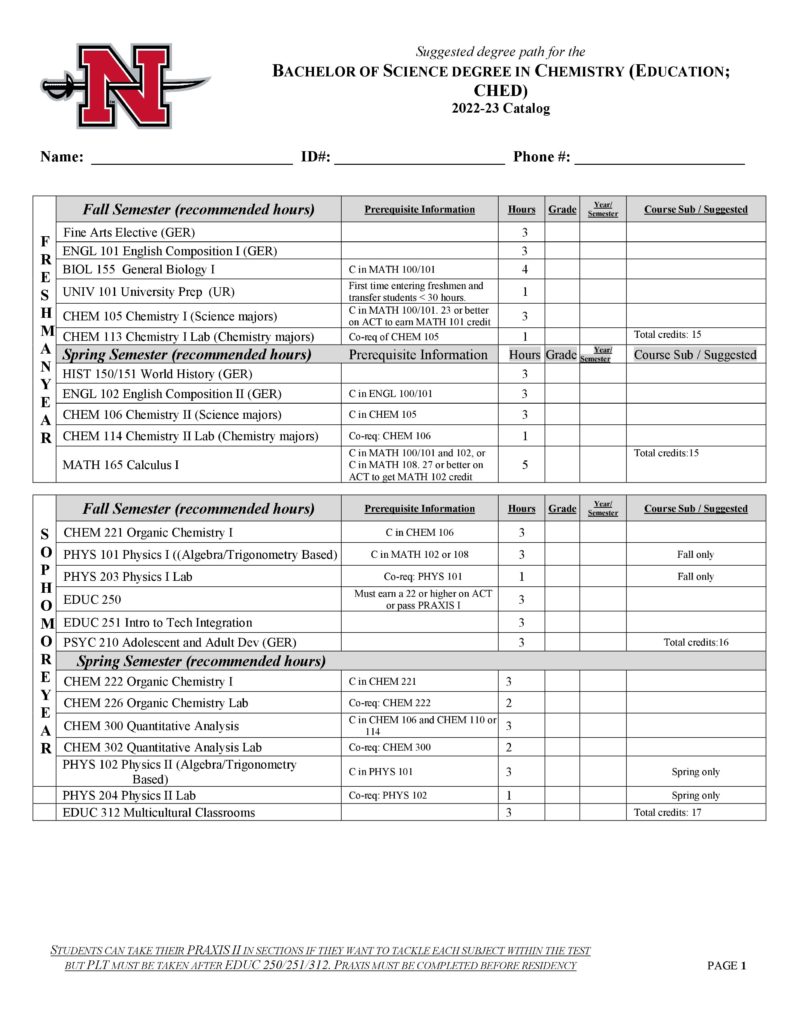
The Department of Physical Sciences offers courses in astronomy, chemistry, geology, physical science and physics and a Bachelor of Science (B. S.) degree in Chemistry with concentrations in the following:
- Professional (CHPR)
- Pre-Medical/Pre-Dental (CHDM)
- Pre-Pharmacy (CHPH)
- Biochemistry (CHBC)
- Chemical Sciences (CHSC)
- Chemistry Education (CHED)
The department also offers one non-degree program:
Pre-Optometry is a two-year non-degree program that prepares a student to enter a school of optometry. Each pre-optometry student will follow the first two years of the chemistry curriculum with appropriate substitutions as approved by the advisor and the head of the Department of Chemistry and Physical Sciences. Most schools of optometry recommend that the student complete three or four years of undergraduate study prior to admission. The typical school of optometry prefers the student with the baccalaureate degree.
For a complete listing of Physical Sciences courses and full course descriptions, check out the catalog entries for Astronomy, Chemistry, Geology, Physical Science and Physics. Find out more about the Physical Sciences curriculum in the undergraduate catalog.
DEGREE PLANS
FACILITIES AND FEATURES
Two computer labs for class use. One has 24 desktop computers, and the other has 8. Students have access to Spartan Student Edition on these computers.
 State-of-the-art and recently renovated labs for chemistry and physics.
State-of-the-art and recently renovated labs for chemistry and physics.
 One large lecture room and several smaller classrooms for pre-lab activities or classroom instruction. Each has a projector, Sympodium, and integrated sound.
One large lecture room and several smaller classrooms for pre-lab activities or classroom instruction. Each has a projector, Sympodium, and integrated sound.
Yusheng Dou is a world-renown computational chemist. He regularly has outside researchers come to collaborate.
 The department hosts free public telescope viewing outreach events for the Nicholls community and Bayou region. These events feature a 150mm Takahashi refractor on a CGE Pro Mount and a Lunt 60mm Hα solar telescope.
The department hosts free public telescope viewing outreach events for the Nicholls community and Bayou region. These events feature a 150mm Takahashi refractor on a CGE Pro Mount and a Lunt 60mm Hα solar telescope.
 The new 400 MHz NMR Spectrometer is used to study how atoms within a molecule are connected together. This powerful tool allows students and faculty to determine the identity and molecular structure of a substance. The main component of this instrument is a superconducting magnet, which is cooled to 4 K with liquid nitrogen and helium. The NMR is primarily used to study organic molecules using 1H, 13C and a variety of two dimensional techniques. However, it is also capable of studying nuclei such as 109Ag, 31P, 15N and 19F with its tunable probe that covers all common NMR frequencies.
The new 400 MHz NMR Spectrometer is used to study how atoms within a molecule are connected together. This powerful tool allows students and faculty to determine the identity and molecular structure of a substance. The main component of this instrument is a superconducting magnet, which is cooled to 4 K with liquid nitrogen and helium. The NMR is primarily used to study organic molecules using 1H, 13C and a variety of two dimensional techniques. However, it is also capable of studying nuclei such as 109Ag, 31P, 15N and 19F with its tunable probe that covers all common NMR frequencies.
 The gas chromatograph – mass spectrometer is used to separate, identify and quantitate volatile components in a mixture. Separation occurs by sample volatilization followed by the use of helium gas to pass the mixture into a column. Within the column, the components separate primarily according to boiling point. After separation, molecules are ionized and sent to a mass analyzer, which produces a mass spectrum. This mass spectrum is used to determine the mass and identity of the substance analyzed. It is used to study volatile organic substances.
The gas chromatograph – mass spectrometer is used to separate, identify and quantitate volatile components in a mixture. Separation occurs by sample volatilization followed by the use of helium gas to pass the mixture into a column. Within the column, the components separate primarily according to boiling point. After separation, molecules are ionized and sent to a mass analyzer, which produces a mass spectrum. This mass spectrum is used to determine the mass and identity of the substance analyzed. It is used to study volatile organic substances.
 HPLC is used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column made up of non-polar material. Each component in the sample interacts slightly differently with the column material, leading to the separation of the components as they flow out the column. This instrument is used to analyze organic compounds.
HPLC is used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column made up of non-polar material. Each component in the sample interacts slightly differently with the column material, leading to the separation of the components as they flow out the column. This instrument is used to analyze organic compounds.
 The ion chromatograph is used to determine anions or cations in water samples. A sample is injected into a stream of eluent of KOH (alternatively methanesulfonic acid (MSA) for cation analysis) and passed through an ion chromatography column. Ions are separated according to their affinity to the ion exchange resin in the column. This instrument is used to analyze both organic and inorganic ions such as nitrates, nitrites, ammonium, chloride, bromide, sulfates, phosphates, lithium, sodium, potassium, calcium and magnesium. The instrument is also able to analyze sugars and amines using electrochemical detection.
The ion chromatograph is used to determine anions or cations in water samples. A sample is injected into a stream of eluent of KOH (alternatively methanesulfonic acid (MSA) for cation analysis) and passed through an ion chromatography column. Ions are separated according to their affinity to the ion exchange resin in the column. This instrument is used to analyze both organic and inorganic ions such as nitrates, nitrites, ammonium, chloride, bromide, sulfates, phosphates, lithium, sodium, potassium, calcium and magnesium. The instrument is also able to analyze sugars and amines using electrochemical detection.
 The gas chromatograph is used to separate, identify and quantitate volatile components in a mixture based on their boiling points. This instrument with its flame ionization detector (FID) is especially useful for analyzing volatile organic substances such as hydrocarbons and alcohols.
The gas chromatograph is used to separate, identify and quantitate volatile components in a mixture based on their boiling points. This instrument with its flame ionization detector (FID) is especially useful for analyzing volatile organic substances such as hydrocarbons and alcohols.
The STEM education journal club brings together faculty to discuss current research in teaching and learning as well as to swap ideas about what works in the classroom.
Generally, we have 4 outside speakers per semester.
 Our students engage with faculty in undergraduate research. They publish and present at conferences. Faculty who mentor students can get a course-release to allow more time for research.
Our students engage with faculty in undergraduate research. They publish and present at conferences. Faculty who mentor students can get a course-release to allow more time for research.
 The flame atomic absorption spectrometer is used to analyze metals in solution based on energy differences between atomic orbitals of an atom. The sample is aspirated into a flame, where the analyte is atomized. A very narrow beam of light is then passed through the flame where atoms of the analyte absorb some of the light, thus decreasing the intensity of the light beam. The intensity of the light varies according to the number of analyte atoms in solution and therefore allows one to determine the amount of analyte in the solution.
The flame atomic absorption spectrometer is used to analyze metals in solution based on energy differences between atomic orbitals of an atom. The sample is aspirated into a flame, where the analyte is atomized. A very narrow beam of light is then passed through the flame where atoms of the analyte absorb some of the light, thus decreasing the intensity of the light beam. The intensity of the light varies according to the number of analyte atoms in solution and therefore allows one to determine the amount of analyte in the solution.
 The fluorescence spectrometer uses light to probe electronic and vibrational states of molecules. Ultraviolet light promotes electrons in certain molecules to an excited state. Light is then emitted as the electron relaxes to lower energy state. The resulting emission spectrum of emission intensity vs. wavelength is then used to characterize the molecule under study.
The fluorescence spectrometer uses light to probe electronic and vibrational states of molecules. Ultraviolet light promotes electrons in certain molecules to an excited state. Light is then emitted as the electron relaxes to lower energy state. The resulting emission spectrum of emission intensity vs. wavelength is then used to characterize the molecule under study.
 Infrared spectroscopy is one of the principal methods employed to study the vibrational characteristics of organic molecules. Molecules absorb infrared light at frequencies that match the vibrational frequency of their bonds. Stronger bonds and light atoms will vibrate at a higher stretching frequency (wavenumber) than do heavier atoms. This allows for the determination of organic functional groups present on a molecule. The spectrometer is equipped with an Attenuated Total Reflection (ATR) accessory allows for minimal sample preparation.
Infrared spectroscopy is one of the principal methods employed to study the vibrational characteristics of organic molecules. Molecules absorb infrared light at frequencies that match the vibrational frequency of their bonds. Stronger bonds and light atoms will vibrate at a higher stretching frequency (wavenumber) than do heavier atoms. This allows for the determination of organic functional groups present on a molecule. The spectrometer is equipped with an Attenuated Total Reflection (ATR) accessory allows for minimal sample preparation.
 The Raman spectrometer is one of the principal instruments used to study the vibrational characteristics of molecules. Incident light is scattered inelastically upon interaction with the molecule’s vibrational states. This loss or gain of energy by the scattered light allows one to probe the characteristics of the molecule. Raman spectroscopy is an especially attractive and powerful tool in environmental and forensic chemical analysis because qualitative and quantitative measurements can be made directly on contaminants in water. Also, the Raman technique offers high information content, non-invasive sampling and minimal sample preparation.
The Raman spectrometer is one of the principal instruments used to study the vibrational characteristics of molecules. Incident light is scattered inelastically upon interaction with the molecule’s vibrational states. This loss or gain of energy by the scattered light allows one to probe the characteristics of the molecule. Raman spectroscopy is an especially attractive and powerful tool in environmental and forensic chemical analysis because qualitative and quantitative measurements can be made directly on contaminants in water. Also, the Raman technique offers high information content, non-invasive sampling and minimal sample preparation.
 The UV-Vis Spectrophotometer measures the wavelengths of ultraviolet and visible electromagnetic radiation that are absorbed by the analyte. The wavelength and amount absorbed is dependent upon the molecular structure of the analyte. This instrument uses a photodiode array detector (PDA) which allows for the complete spectrum of a molecule to be obtained in a matter of seconds.
The UV-Vis Spectrophotometer measures the wavelengths of ultraviolet and visible electromagnetic radiation that are absorbed by the analyte. The wavelength and amount absorbed is dependent upon the molecular structure of the analyte. This instrument uses a photodiode array detector (PDA) which allows for the complete spectrum of a molecule to be obtained in a matter of seconds.
 The electrochemical work station is used to study the electrochemical properties of metals and organic molecules in solution, using coulometric and voltammetric techniques. One common technique is cyclic voltammetry (CV), which is performed by cycling the potential of a working electrode, and measuring the resulting current. CV, in addition to determining amounts of analyte present, is also a powerful tool for studying electron transfer reactions.
The electrochemical work station is used to study the electrochemical properties of metals and organic molecules in solution, using coulometric and voltammetric techniques. One common technique is cyclic voltammetry (CV), which is performed by cycling the potential of a working electrode, and measuring the resulting current. CV, in addition to determining amounts of analyte present, is also a powerful tool for studying electron transfer reactions.
CONTACT INFO
138 Kilgen Hall
906 E. First St.
Thibodaux, LA 70310